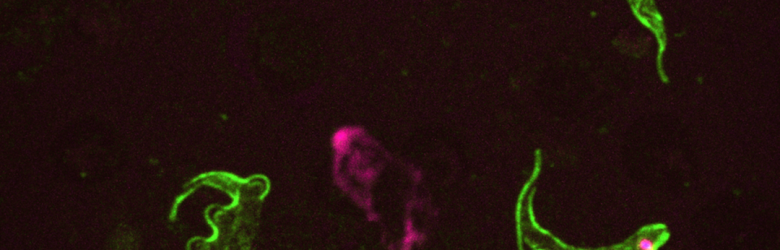
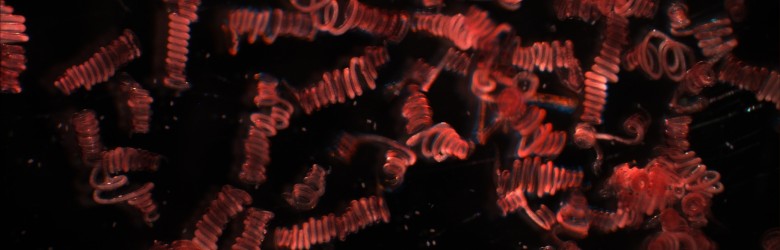
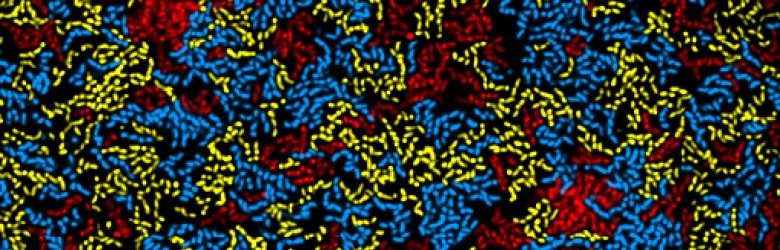
To understand the evolutionary biology of infectious diseases, we need to study both within-host (e.g., immunological) and between-host (e.g., transmission-related) processes. Why? We must study both scales because we know that parasites must succeed on both scales if they are going to spread and persist in host populations.
The logic is as follows. A parasite that outwits its host and escapes clearance by the immune system can only be successful evolutionarily — i.e., can only gain genetic representation in the next generation of parasites — if it is also transmitted from one host to the next. In other words, immune escape that leads to a transmission dead-end will get a parasite nowhere! And conversely, a parasite that excels at transmission (traveling from one host to the next, whether via a sneeze or an insect vector) yet fails to survive immune attack in the next host will also be an evolutionary dud.
So we as scientists must understand parasites across the whole life cycle if we are to understand and predict the evolution of parasites over time and in response to medical interventions.